"One hundred
and fifty years ago humans started a grand,
Jump to: [Introduction]
[Carbon Accounting] [Cycling]
[Controls] [Fixes] [Self-Test]
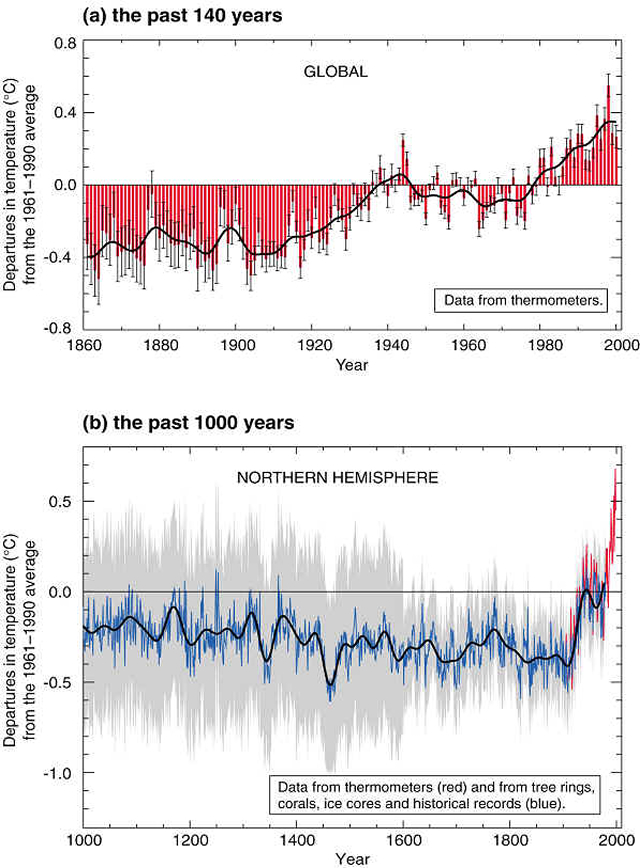
Although we don't usually think of carbon in our day to day lives, carbon plays the central role in the "reduction-oxidation battlefield" that we call life. On the one side of the battlefield you have the photosynthetic organisms that use light energy and atmospheric CO2 to produce a pool of reduced organic compounds with high potential energy, plus a reservoir of oxygen. On the other side of the battlefield you have the heterotrophic creatures, like ourselves, that are trying to release this stored energy by oxidizing the reduced organic compounds, consuming O2 and adding CO2 to the atmosphere in the process. What humans have done is to accelerate this oxidation through fossil fuel burning and land use changes, so much so that we have started a grand, uncontrolled experiment with carbon on earth. We now recognize that this experiment will change our climate, and the potential effects on people's lives have stimulated some of the largest public and policy debates of any scientific topic today. In this lecture we will learn more about the carbon cycle, how it functions on earth, and how it is controlled by natural and anthropogenic processes. The carbon cycle has been linked to the changes in climate that we have recently observed on earth, especially the increases in temperature shown in Figure 1 (below). The Intergovernmental Panel on Climate Change (IPPC) in its most recent report (2002) states that "There is new and stronger evidence that most of the warming observed over the last 50 years is attributable to human activities”, and that “…most of the observed warming over the last 50 years is likely to have been due to the increase in greenhouse gas concentrations”.
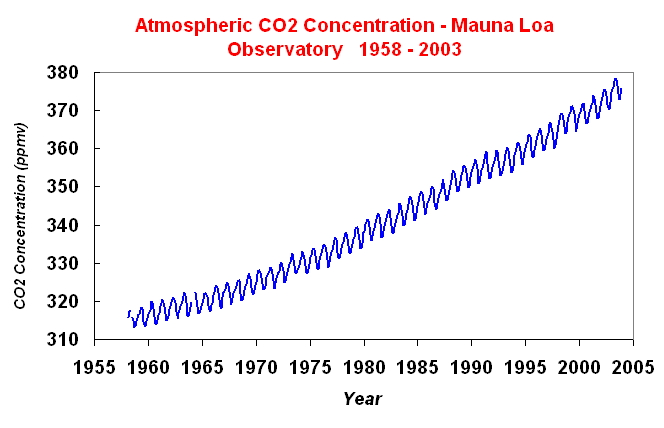
Figure 1. Changes in the Earth's surface temperature for the last 140 (above) and the change in temperature for the northern hemisphere for the last 1000 years (below). These changes in CO2 are shown below (Figure 2) for the record of atmospheric concentration at the Mauna Loa Observatory in Hawaii. The concentrations have been increasing steadily since measurements began in 1958, and other records indicate that these increases in atmospheric CO2 began in the middle 1800s at the start of the human industrial revolution.
Figure 2. Changes in the concentration of CO2 over time in the Earth's atmosphere (above; from D. Keeling). In addition to the changes in CO2, human influence has contributed to changes in other greenhouse gases in the atmosphere as well, as shown in Figure 3 below. It is this evidence of consistent changes in greenhouse gases correlated with human activity which has led to the IPCC conclusions that humans are increasing greenhouse gas concentrations and that those gases are in large part driving climate change.
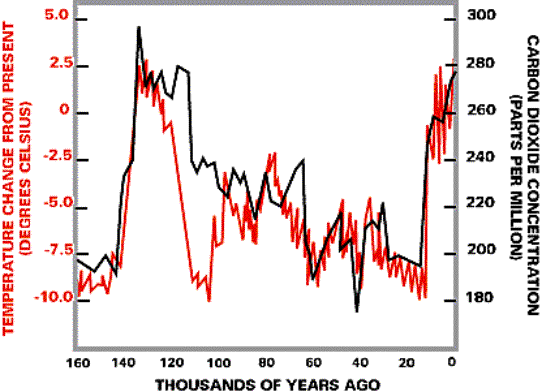
Figure 3. Relationship between increases in greenhouse gases over time. Note the consistent increases in the mid 1800s at the start of the industrial revolution. There is also a strong correlation between CO2 concentration in the atmosphere and global temperatures for the past. Figure 4 below shows this correlation over the last 160,000 years, and it is clear that the temperature and CO2 concentrations in the atmosphere are linked. This is consistent with our current understanding of how the greenhouse effect operates.
Figure 4. Plot showing how CO2 concentrations (estimated from measurements of CO2 trapped in ice cores) are correlated with global temperature (estimated from indicators such as isotopes in organic matter of ocean sediments) over the last 160,000 at least. Although the CO2 concentrations shown in Figure 4 are all below today's concentration of ~375 ppm, there have been times on earth in the past when the CO2 concentration of the atmosphere has also been greater than it is today (see Figure 6). If it has been greater, then one might ask "why are we so worried, the CO2 concentrations were greater than they are today and we still survived?" The answer to that question lies in the fact that the rate of change of CO2 in the atmosphere is faster today than at anytime in earth's history. It is this rapid increase in CO2, not necessary the final CO2 concentration that may be reached, which is causing much of our concern. For example, because organisms (and certainly all of the human cultures on earth) have never been exposed to such rapid rates of CO2 increase, we don't know how they will respond and whether they will be able to adapt quickly enough to survive. These are the questions that science and society are struggling with today. While we know that CO2 concentrations are increasing, there have been several plans or ideas on how to control them "naturally", such as plant more trees to take up the excess CO2. Later in the lecture we will examine two of these ideas and determine if they could be real solutions to global warming. In order to understand the carbon cycle we must understand first the "accounting" of where the pools of carbon are located, second the "pathways and cycling" of how much and how fast the carbon is moving from one pool to another, and third the "controls" on how much carbon is cycled. There are several different forms of carbon that we have to keep track of in learning about the carbon cycle. The main forms are (a) Inorganic-C in rocks (such as bicarbonate and carbonate); (b) organic-C (such as found in organic plant material); and (c) carbon gases such as CO2, (carbon dioxide), CH4, (methane), and CO (carbon monoxide). "Carbon cycling" is really all about the movement of C from one of these forms to another form. Table 1 gives an accounting of where
these different forms of carbon are located on earth (note that 1015
g = 1 billion tons = 1 gigaton = 1 Pedagram):
* In the atmosphere, CO2 is 99.6% of the total (i.e., the amount of CH4 is small).
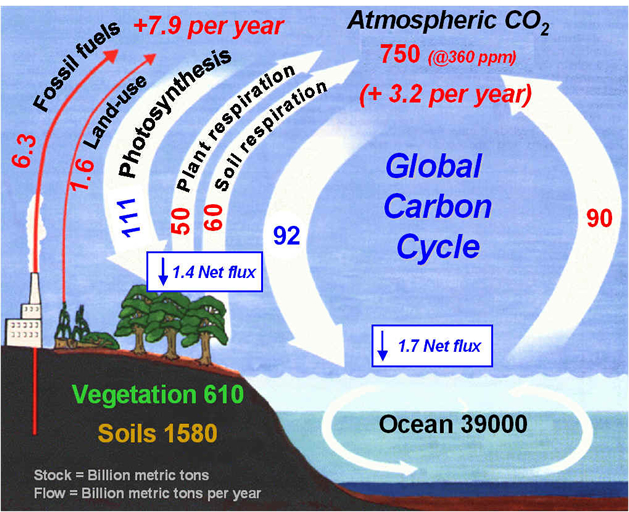
Figure 5. Pathways, pools, and fluxes in the global carbon cycle. Note that the actual numbers vary slightly with different estimates, and are used here only as guides to the levels of fluxes and pools. There are several pathways in the carbon cycle that are of particular importance (Figure 5 above). The main pathways to and from the atmosphere are diffusion into and out of the ocean, photosynthesis which consumes CO2 from the atmosphere (an output from the atmosphere), respiration which produces CO2 (an input to the atmosphere), and the burning of fossil fuels and biomass which also produces an input of CO2 to the atmosphere. Although the largest storage of carbon is in rocks (Table 1), the largest magnitudes of fluxes don't involve the rock pool directly on short time periods. The magnitudes of C fluxes are as follows (all in 1015 g of C per year): ·
Ocean uptake = 1.7 (x 1015 g C / yr)
Mass Balance As discussed in the lecture on Ecosystems, we can
use biogeochemical principles to construct a mass balance for
the atmosphere by knowing that the amount of C in the atmosphere increases
by 3.2 x 1015 g C /yr, by knowing that the internal change in the
atmosphere is zero, and by knowing the other fluxes into and out of the
atmosphere:
Given this analysis we find that there is an imbalance -- we need an additional "sink" of -2.2 (3 - 5.2) x1015 g C to balance the global C budget. In other words, we are "missing" over 2 billion tons of C each year; this shows how incomplete our understanding of the global carbon cycle is at present. Although it is possible that this missing sink could be found in any of the major pools of carbon on earth, it is most likely that the pools with a shorter residence time such as vegetation, soils or the ocean (compared to rocks) are most important. Recent models indicate that terrestrial ecosystems are the most likely repository of this carbon. In order to fully understand how the carbon cycle operates, and to gain a better understand of issues such as the missing sink of C and slowing the rate of C increase in the atmosphere, we must examine the controls on carbon movement. There are several dominant controls on the flux rates of carbon between different carbon pools on earth, including the influence of volcanic activity and rock weathering, which are most important on long time scales. Human influence on the global carbon cycle has been very recent, but has altered the C-cycle in a dramatic way by adding CO2 and CH4 to the atmosphere. (A). Volcanic activity:
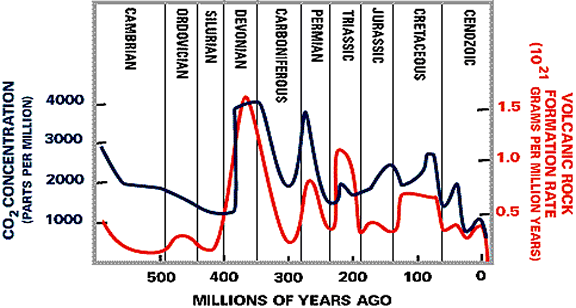
CO2 concentrations in the atmosphere are related to volcanic activity, as Figure
6 illustrates.
This shows that the concentration of CO2 has varied over a large range
in our atmosphere in the geologic past. When volcanic activity is high the
CO2 concentration in the atmosphere is also high due to the release of CO2 from
volcanoes into the atmosphere. Large, single volcanic eruptions today have
much less effect on the atmospheric CO2 concentration, but can release particles
into the atmosphere that can cause a slight (but temporary) cooling of the
Earth's surface.
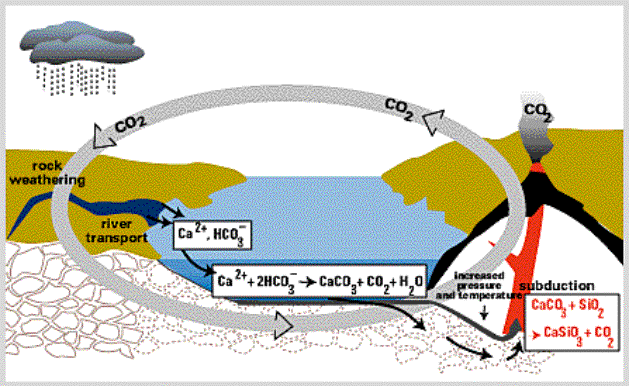
Figure 6. The relationship between CO2 concentration in the atmosphere and volcanic activity on earth. (B). Rock weathering: CO2 concentrations have been limited over geologic time to a range of about 200 - 6000 ppm in the atmosphere due to weathering of rocks and the formation of carbonate minerals. Figure 7 shows that CO2 in the atmosphere is consumed in the weathering of many rocks. This weathering produces bicarbonate (HCO3-), a form of inorganic carbon, and calcium (Ca2+) that are then transported in river water to the oceans. Once in the oceans the calcium and bicarbonate are combined by organisms to form calcium carbonate, the mineral that is found in shells. This calcium carbonate mineral is buried in the sediments, where eventually it comes under great temperature and pressure and is melted during the process of "subduction", when one tectonic plate moves under another. The melted rock rises to the surface in the form of magma and is released back to the surface of the earth through volcanoes. This high-temperature process also converts some of the calcium carbonate back to CO2, which is released to the atmosphere to begin the cycle over again.
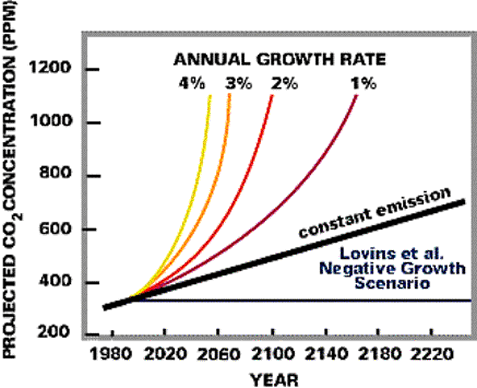
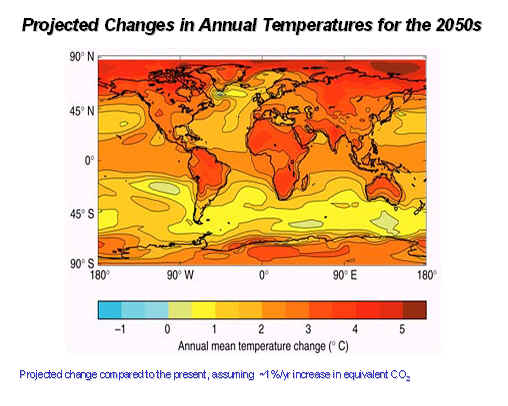
Figure 7. The global carbon cycle from the perspective of its control by weathering. (C.) Human Activity: Humans are changing the CO2 cycle by burning fossil fuels and biomass (e.g., forests), and also by converting landscapes that store a lot of carbon (e.g., forests) into agricultural or urban landscapes that store very little carbon. These increases in CO2 are linked to increased warming in our atmosphere, as discussed above; for example, the CO2 level in our atmosphere was only about 280 ppm before human-induced changes began occurring about 150 years ago. Since humans started to pump CO2 into the atmosphere there has been a large increase in atmospheric CO2, and this increase will undoubtedly continue in the future as indicated by the following graph (Figure 8). The CO2 concentration in our atmosphere will likely double with only a modest 1% growth over the next 100 years (note that global population growth is much greater than that at the present). This rise in CO2 will increase the Earth's temperature as shown in Figure 9, and will impact the climate that we actually "feel" in the future (Figure 10).
Figure 8 (above). Projected CO2 increases in the atmosphere given different scenarios of population growth.
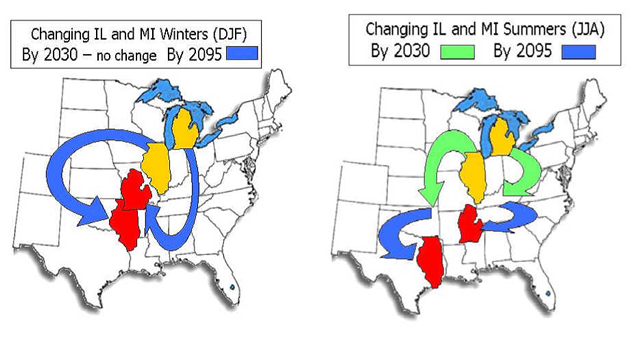
Figure 9 (above). Projected global surface temperatures by 2050. Figure 10 (below). Illustration of the impacts of climate warming on the climate that we "feel" in Michigan and Illinois. By 2095, a typical Michigan summer will "feel" like a summer in northern Mississippi today (Wuebbles and Hayhoe, unpublished).
An important point to consider is that even if we stopped our emissions of CO2 today, we would still experience climate change because of what has already been set in motion. This is because the lifetime (residence time) of several important greenhouse gases is fairly long in the atmosphere; thus, even if human-related emissions of CO2 were totally halted right now, the atmospheric levels of CO2 would decay very slowly over the coming decades. These long atmospheric lifetimes, and the biological realities of how life adapts to rapid climate change, add an urgency to the need to develop responses to reduce potential adverse impacts of climate change. Meeting the challenges associated with the combined impacts of climate change and direct human pressures on the environment involves three principal approaches:
All of these approaches are discussed in detail in later semesters of our Global Change program (Global Change II & III), but in this lecture we will discuss the scientific aspects of several ideas about how we might slow the increase of CO2 in the atmosphere. One of these potential "fixes" was known as the "Geritol Fix" (Geritol is a vitamin pill containing iron - ask your parents, they'll remember...) and it involves the fact that algal growth in the southern oceans is limited by iron (Fe). If you add Fe you stimulate growth and the uptake of CO2 from the atmosphere by algae. In a careful biogeochemical analysis, however, this idea proved to be untenable because the algae would eventual run out of other limiting nutrients such as nitrogen and phosphorus. The Geritol Fix could at most reduce our atmospheric CO2 concentrations by 10%. Another idea has been that because land plants take up CO2 during photosynthesis, and CO2 stimulates their growth, we could solve the whole greenhouse gas problem by planting more trees. Once again, this solution runs up against some biogeochemical realities. For example, where will the plants find the extra nitrogen in order to grow? We don't know the answer to this question, but this active area of research is gaining more knowledge on how the carbon and nitrogen cycles are linked, especially with respect to plants taking up excess CO2 in the atmosphere. In addition to planting trees to take up more CO2, there are different ways in which agriculture may also contribute to the solution of reducing the amount of CO2 in our atmosphere. In the end (and the take-home message for today's lecture), we must recognize that "There are no magic fixes for the CO2 problem" -- a variety of solutions will be required to minimize the impacts of our altered carbon cycle on earth's ecosystems and inhabitants, which will be talked about in the next lecture on Great Lakes Climate Change.
All materials © the Regents of the University of Michigan unless noted otherwise. |