Climate Change, Radiative Forcing, Aerosols, and Global Warming Potential of Gases in Our Atmosphere
| Natural Climate Change | Radiative Forcing | Aerosols | Global Warming Potential | Summary |
Driving Questions:
- What has controlled past changes in Earth's temperature?
- What processes lead to temperature change?
- What gases and aerosols comprise the atmosphere and how do they influence the temperature?
- What are the global warming potentials for greenhouse gases?
1. Natural Climate Change
We believe that the temperature of the earth has varied wildly over the evolution of the earth. Figure 1 shows an estimate of temperature changes as complied by Scotese. So how can it be that the climate has changed so over the ages and what processes could lead to these changes?
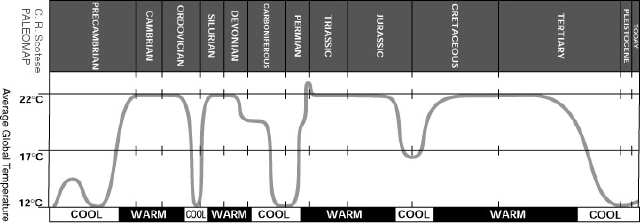
Figure 1. Estimated changes in global temperature.
The processes for changing climate naturally over long timescales include:
-
Plate tectonics
-
Long-term carbon cycle
-
Solar Variations (sun's luminosity), and
-
Orbital Variations (which will be discussed in a later lecture)
2. Radiative Forcing
The temperature of the Earth's surface and atmosphere are dictated by
a balance between incoming energy and outgoing energy. When more energy
is received than lost, temperatures rise. The Earth's surface, for example,
absorbs radiation from the Sun. This energy is then redistributed by the
atmospheric and oceanic circulations and radiated back to space at longer
(infrared) wavelengths. For the annual mean and for the Earth as a whole,
the incoming solar radiation energy is balanced approximately by the outgoing
terrestrial radiation. Any factor that alters the radiation received from
the Sun or lost to space, or that alters the redistribution of energy
within the atmosphere and between the atmosphere, land, and ocean, can
affect climate. A change in the net radiative energy available to the
global Earth-atmosphere system is termed a radiative forcing. Positive
radiative forcings tend to warm the Earth’s surface and lower atmosphere.
Negative radiative forcings tend to cool them.
The Greenhouse Effect
Increases in the concentrations of greenhouse gases will reduce the efficiency
with which the Earth’s surface radiates to space. More of the outgoing
terrestrial radiation from the surface is absorbed by the atmosphere and
re-emitted at higher altitudes and lower temperatures. This results in
a positive radiative forcing that tends to warm the lower atmosphere and
surface. Because less heat escapes to space, this is the enhanced greenhouse
effect – an enhancement of an effect that has operated in the
Earth’s atmosphere for billions of years due to the presence of naturally
occurring greenhouse gases: water vapor, carbon dioxide, ozone, methane
and nitrous oxide. The amount of radiative forcing depends on the size
of the increase in concentration of each greenhouse gas, the radiative properties of the gases involved (indicated by their global warming
potential), and the concentrations of other greenhouse gases already
present in the atmosphere. Further, many greenhouse gases reside in the
atmosphere for centuries after being emitted, thereby introducing a long-term
commitment to positive radiative forcing.
3. Aerosols
Anthropogenic aerosols (microscopic airborne particles or droplets) in the troposphere, such as those derived from fossil fuel and biomass burning, can reflect solar radiation, which leads to a cooling tendency in the climate system. Because it can absorb solar radiation, black carbon (soot) aerosol tends to warm the climate system.
Sources of aerosols include: Biomass combustion (fires); Wind-blown dust; Salt from sea spray; Volcanoes; and Industrial pollution. Aerosols have both a direct and an indirect effect on albedo in the atmosphere or on the ground surface.
1. Direct effect: aerosols reflect and scatter incoming solar radiation, causing an increase in albedo (leads to negative radiative forcing = net cooling).
2. Indirect effect: aerosols influence cloud properties (albedo, height, droplet size, causing net cooling). A particularly important indirect effect is that sulfate aerosols derived from sulfur gases emitted from volcanoes and in pollution, act as cloud condensation nuclei (provide a tiny nucleus for water vapor to condense around and form a water droplet) and in doing so they increase the thickness or optical depth of clouds, increasing their albedo. A second important indirect effect is that the black carbon in soot that comes from pollution or burning, will decrease the albedo of surfaces and especially of ice or snow (leads to a net warming).
Changes in aerosol concentrations can alter cloud amount and cloud reflectivity through their effect on cloud properties and lifetimes. In most cases, tropospheric aerosols tend to produce a negative radiative forcing and a cooler climate. They have a much shorter lifetime (days to weeks) than most greenhouse gases (decades to centuries), and, as a result, their concentrations respond much more quickly to changes in emissions. Volcanic activity can inject large amounts of sulphur-containing gases (primarily sulfur dioxide) into the stratosphere, which are transformed into sulfate aerosols. Individual eruptions can produce a large, but transitory, negative radiative forcing, tending to cool the Earth’s surface and lower atmosphere over periods of a few years. For example, the large volcanic eruption of Mt. Pinatubo in the Phillipines in 1991 caused the release of sulfur dioxide that produced sulfate aerosols, which stayed in Earth's atmosphere for over 2 years and caused a short-term cooling of 0.5 degC in Earth's surface temperature.
Changes in Radiative Forcing
When radiative forcing changes, the climate system responds on various
time-scales. The longest of these are due to the large heat capacity of
the deep ocean and dynamic adjustment of the ice sheets. This means that
the transient response to a change (either positive or negative) may last
for thousands of years. Any changes in the radiative balance of the Earth,
including those due to an increase in greenhouse gases or in aerosols,
will alter the global hydrological cycle and atmospheric and oceanic circulation,
thereby affecting weather patterns and regional temperatures and precipitation.
4. Global Warming Potential
The Global Warming Potential (GWP) of a greenhouse gas is the ratio of global warming, or radiative forcing – both direct and indirect – from one unit mass of a greenhouse gas to that of one unit mass of carbon dioxide over a period of time. Hence this is a measure of the potential for global warming per unit mass relative to carbon dioxide. This allows for different gases to be compared on the same, level playing field.
The Global Warming Potential of any gas is determined by three main factors:
1. The amount of absorption of infrared radiation by a gas
2. The spectral location of its absorbing wavelengths (if it absorbs in a range of high longwave emission from Earth, its GWP is higher)
3. The atmospheric lifetime of the gas (the longer it stays in the atmosphere, the greater the GWP)
Atmospheric Lifetime
The residence time of a gas in the atmosphere (the residence time, Rt, is the average time that any material spends in a pool or location before leaving) is called its Atmospheric Lifetime. These lifetimes range from months to centuries, and are important in determining the overall GWP of a gas. But they also provide information on the behavior of a system, and the residence time of a gas has implications for its overall effect. For example, we see that CO2 has a lifetime of 50-200 years in the atmosphere. The implication of that long residence time is that even if we were stop human emissions of CO2 into the atmosphere right now, our future climate would still be affected by the amount of CO2 we have already put into the atmosphere. It is like raking leaves into a big pile - once you stop raking the pile doesn't get any bigger, but, it also doesn't go away immediately. The same thing occurs with CO2 in the atmosphere -- because it has a long residence time, it will persist and continue to warm our planet, creating a "legacy effect" of past CO2 emissions that future generations will bear.
GWP Comparisons
Global Warming Potentials are presented in Table 1 for an expanded set of gases compared to what was shown in lecture. GWPs are a measure of the relative radiative effect of a given substance compared to CO2, integrated over a chosen time horizon. New categories of gases in Table 1 include fluorinated organic molecules, many of which are ethers that are proposed as halocarbon substitutes. Some of the GWPs have larger uncertainties than that of others, particularly for those gases where detailed laboratory data on lifetimes are not yet available. The direct GWPs have been calculated relative to CO2 using an improved calculation of the CO2 radiative forcing, the SAR response function for a CO2 pulse, and new values for the radiative forcing and lifetimes for a number of halocarbons. Indirect GWPs, resulting from indirect radiative forcing effects, are also estimated for some new gases, including carbon monoxide. The direct GWPs for those species whose lifetimes are well characterized are estimated to be accurate within ±35%, but the indirect GWPs are less certain.
| Table 1. Direct Global Warming Potentials (GWPs) relative to carbon dioxide (for gases for which the lifetimes have been adequately characterized). GWPs are an index for estimating relative global warming contribution due to atmospheric emission of a kg of a particular greenhouse gas compared to emission of a kg of carbon dioxide. GWPs calculated for different time horizons show the effects of atmospheric lifetimes of the different gases. | |||||
| Lifetime | Global Warming Potential | ||||
| (years) | (Time Horizon in Years) | ||||
| GAS | 20 yrs | 100 yrs | 500 yrs | ||
| Carbon Dioxide | CO2 |
50-200
|
1
|
1
|
1
|
| Methane | CH4 |
12.0
|
62
|
23
|
7
|
| Nitrous Oxide | N2O |
114
|
275
|
296
|
156
|
| Chlorofluorocarbons |
|
|
|
|
|
| CFC-11 |
55
|
4500
|
3400
|
1400
|
|
| CFC-12 |
116
|
7100
|
7100
|
4100
|
|
| CFC-115 |
550
|
5500
|
7000
|
8500
|
|
| Hydrofluorocarbons |
|
|
|
|
|
| HFC-23 | CHF3 |
260
|
9400
|
12000
|
10000
|
| HFC-32 | CH2F2 |
5
|
1800
|
550
|
170
|
| HFC-41 | CH3F |
2.6
|
330
|
97
|
30
|
| HFC-125 | CHF2CF3 |
29
|
5900
|
3400
|
1100
|
| HFC-134 | CHF2CHF2 |
9.6
|
3200
|
1100
|
330
|
| HFC-134a | CH2FCF3 |
13.8
|
3300
|
1300
|
400
|
| HFC-143 | CHF2CH2F |
3.4
|
1100
|
330
|
100
|
| HFC-143a | CF3CH3 |
52
|
5500
|
4300
|
1600
|
| HFC-152 | CH2FCH2F |
0.5
|
140
|
43
|
13
|
| HFC-152a | CH3CHF2 |
1.4
|
410
|
120
|
37
|
| HFC-161 | CH3CH2F |
0.3
|
40
|
12
|
4
|
| HFC-227ea | CF3CHFCF3 |
33
|
5600
|
3500
|
1100
|
| HFC-236cb | CH2FCF2CF3 |
13.2
|
3300
|
1300
|
390
|
| HFC-236ea | CHF2CHFCF3 |
10
|
3600
|
1200
|
390
|
| HFC-236fa | CF3CH2CF3 |
220
|
7500
|
9400
|
7100
|
| HFC-245ca | CH2FCF2CHF2 |
5.9
|
2100
|
640
|
200
|
| HFC-245fa | CHF2CH2CF3 |
7.2
|
3000
|
950
|
300
|
| HFC-365mfc | CF3CH2CF2CH3 |
9.9
|
2600
|
890
|
280
|
| HFC-43-10mee | CF3CHFCHFCF2CF3 |
15
|
3700
|
1500
|
470
|
| Fully fluorinated species |
|
|
|
|
|
| SF6 |
3200
|
15100
|
22200
|
32400
|
|
| CF4 |
50000
|
3900
|
5700
|
8900
|
|
| C2F6 |
10000
|
8000
|
11900
|
18000
|
|
| C3F8 |
2600
|
5900
|
8600
|
12400
|
|
| C4F10 |
2600
|
5900
|
8600
|
12400
|
|
| c-C4F8 |
3200
|
6800
|
10000
|
14500
|
|
| C5F12 |
4100
|
6000
|
8900
|
13200
|
|
| C6F14 |
3200
|
6100
|
9000
|
13200
|
|
| Ethers and Halogenated Ethers |
|
|
|
|
|
| CH3OCH3 |
0.015
|
1
|
1
|
<<1
|
|
| HFE-125 | CF3OCHF2 |
150
|
12900
|
14900
|
9200
|
| HFE-134 | CHF2OCHF2 |
26.2
|
10500
|
6100
|
2000
|
| HFE-143a | CH3OCF3 |
4.4
|
2500
|
750
|
230
|
| HCFE-235da2 | CF3CHClOCHF2 |
2.6
|
1100
|
340
|
110
|
| HFE-245fa2 | CF3CH2OCHF2 |
4.4
|
1900
|
570
|
180
|
| HFE-254cb2 | CHF2CF2OCH3 |
0.22
|
99
|
30
|
9
|
| HFE-7100 | C4F9OCH3 |
5
|
1300
|
390
|
120
|
| HFE-7200 | C4F9OC2H5 |
0.77
|
190
|
55
|
17
|
| H-Galden 1040x | CHF2OCF2OC2F4OCHF2 |
6.3
|
5900
|
1800
|
560
|
| HG-10 | CHF2OCF2OCHF2 |
12.1
|
7500
|
2700
|
850
|
| HG-01 | CHF2OCF2CF2OCHF2 |
6.2
|
4700
|
1500
|
450
|
Summary
- Greenhouse gases selective absorb infrared radiation, thus trapping energy in the atmosphere.
- Aerosols (except soot or black carbon) have a net cooling effect on the atmosphere because they increase Earth's albedo, reflecting incoming radiation back out to space. Soot (black carbon) can decrease surface albedo, especially for snow or ice, and thus slightly warm the Earth.
- The atmosphere radiates energy to the surface at an average rate greater than the rate of incoming solar radiation.
- Each greenhouse gas is characterized by its atmospheric lifetime and global warming potential.
All materials ©
the Regents of the University of Michigan unless noted otherwise.